Matter in Our Surroundings - NCERT Questions
Matter in our surroundings is the 1st chapter from NCERT Chemistry textbook of class 9. Here in this chapter, we will first start by discussing the introduction of the chapter, followed by the chapter end questions and answers.
Introduction
From the perspective of your CBSE Class 9 Science examination, NCERT Solutions for Class 9 Science (Chemistry), Chapter 1 Matter in Our Surroundings, is an essential study resource.
You will be better able to comprehend the key ideas in the chapter by studying the thorough NCERT Solutions for Class 9 Chemistry to each exercise question provided here.
Chapter 9, Matter in our surroundings is a crucial topic in the science textbook for class 9 and serves as the foundation for many later-level concepts. With the help of determined experts with in-depth conceptual knowledge and years of experience, solutions are created.
The NCERT Solutions' content has also been updated to reflect the most recent NCERT syllabus and make it easily understandable as well as available for students via Champstreet.
Which of the following are matter?
Chair, air, love, smell, hate, almonds, thought, cold, cold drink, smell of perfume.
Chair, air, smell, almonds, cold drink and smell of perfume
Q 2. Give reasons for the following observation:
The smell of hot sizzling food reaches you several metres away, but to get smell from cold food, you have to go close.
This happens because rate of diffusion of gas increases with increase in temperature. In case of hot food, diffusion of smell is faster whereas in case of cold food, diffusion is slower.
Q 3.A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
SOLUTION:The particles of water are held together by forces of attraction. It is the reason that the diver is able to cut through water in a swimming pool.
Q 4. (A) Tabulate the differences in the characteristics of states of matter.
(B) Comment upon the following : rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density
(A)
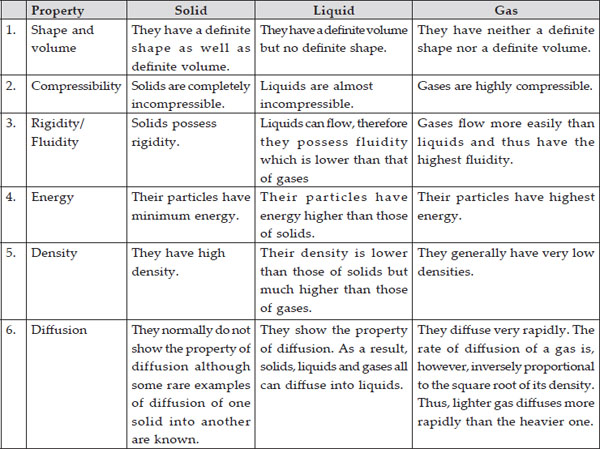
(B) (i) Rigidity : It is the property which helps a substance to retain its shape when force is applied to it. Solids are rigid while gases and liquids are not.
(ii) Compressibility : The property due to which the particles of matter can be compressed or reduced in volume by applying force or pressure. Gases are highly compressible.
(iii) Fluidity : It is the tendency of a substance to flow. Liquids and gases possess fluidity while solids are rigid.
(iv) Filling a gas container : The molecules of a gas move in all directions and due to negligible interparticle force of attraction can fill the container.
(v) Shape : Solids have definite shape whereas liquids take the shape of the container in which they are placed and gases do not have any shape.
(vi) Kinetic energy : It is the energy possessed by the particles due to their motion. The particles of a gas have maximum kinetic energy due to free motion of gas particles in all directions. Solids have minimum kinetic energy due to least movement of particles.
(vii) Density : Density is the mass of a substance per unit volume. Solids have highest density since their molecules are closely packed.
What are the characteristics of the particles of matter?
SOLUTION: The characteristics of the particles of matter are following :
(A) Matter consists of tiny particles which cannot be seen by an individual with naked eye.
(B) The particles of matter have spaces between them.
(C) The particles of matter attract each other due to intermolecular forces of attraction. The forces of attraction are maximum in solids and minimum in gases. Liquids have intermolecular forces in between solids and gases.
(D) The particles of matter are not stationary but are continuously moving.
(E) The intermolecular forces decrease with the increase in intermolecular spaces and vice-versa.
(F) Kinetic energy of the molecules increases with the rise in temperature.
The mass per unit volume of a substance is called density.
(density = mass/volume)
Arrange the following in order of increasing density – air, exhaust from chimneys, honey, water, chalk, cotton and iron.
The increasing order of density is
air < exhaust from chimney < cotton < water < honey < chalk < iron.
Give reasons.
A. A gas fills completely the vessel in which it is kept.
B. A gas exerts pressure on the walls of the container.
C. A wooden table should be called a solid.
D. We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
(A) The particles of a gas are constantly moving in all the directions with different speeds. Therefore they do not have a fixed volume and hence completely fill the vessel in which they are kept.
(B) The molecules of a gas are free to move randomly in all directions. During their motion, they collide with one another and also with the walls of the container. The constant bombardment of the molecules on the walls of the container exerts a steady force. The force acting per unit area on the walls of the container is called pressure. Thus, gases exert pressure.
(C) A wooden table is called solid because it has a definite mass, volume and shape.
(D) In air, there is lot of empty space between the molecules and the forces between the particles are almost negligible. Hence, we can move our hand in air. However we cannot move our hand in a solid block because there are strong forces of attractions between particles in a solid and there is no empty space between them.
Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why?
SOLUTION:When water freezes to form ice, some empty spaces are created. As a result, volume increases for the same mass of water. In other words, mass per unit volume or density of ice is lower than that of water and hence ice floats over water.
Q 9. Convert the following temperatures to celsius scale :
A. 300 K
B. 573 K.
(A) Temperature in °C
= Temperature in kelvin – 273
= 300 – 273 = 27°C
(B) Temperature in °C = 573 – 273 = 300°C
What is the physical state of water at :
A. 250°C
B. 100°C ?
(A) Physical state of water at 250°C is gaseous state because the boiling point of water is 100°C. Therefore at a temperature higher than its boiling point, it exists as gas.
(B) At 100°C both liquid and gaseous states are present. These are in a state of equilibrium. So at 100°C both liquid water and vapours are present.
For any substance, why does the temperature remain constant during the change of state?
SOLUTION:The temperature remains constant during the change of state because the heat supplied during the change is used up in overcoming the intermolecular forces between the particles of the state.
Q 12.Suggest a method to liquefy atmospheric gases.
SOLUTION:In order to liquefy a gas, the constituent particles or molecules have to be brought closer. The atmospheric gases can be liquefied either by increasing pressure or by decreasing temperature.
Q 13.Why does a desert cooler cool better on a hot dry day?
SOLUTION:The cooling in a desert cooler is caused by the evaporation of water. A desert cooler cools better on a hot and dry day because the higher temperature on a hot day and the dryness of air (low humidity of air) increases the rate of evaporation of water. Hence due to increased rate of evaporation of water, a desert cooler cools better on a hot and dry day.
Q 14.How does the water kept in an earthen pot (matka) become cool during summer?
SOLUTION:An earthen pot (matka) has many small pores. Water seeps out through them and evaporates from the surface of the pot. The energy needed for evaporation is taken from the water kept in the earthen pot. As a result, water kept in earthen pot becomes cool.
Q 15.Why does our palm feel cold when we put some acetone or petrol or perfume on it?
SOLUTION:Both acetone and perfume are low boiling liquids. When they are poured on the palm, they evaporate readily and for this change of state they take the energy from the palm and we get a cooling sensation.
Q 16.Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
SOLUTION:We are able to sip hot tea faster from a saucer rather than a cup because a saucer has a greater surface area. As a result, rate of evaporation increases.
Q 17.What type of clothes should we wear in summer?
SOLUTION:We should wear cotton clothes in summer. During summer, we perspire more because of the mechanism of our body which keep us cool. Cotton, being a good absorber of water helps in absorbing the sweat and exposing it to the atmosphere for easy evaporation.
Q 18. Convert the following temperatures to Celsius scale :
A. 293 K
B. 470 K
(A) We know that, temperature in
°C = K – 273
= 293 – 273 = 20°C
(B) °C = K – 273 = 470 – 273 = 197°C
Convert the following temperatures to the Kelvin scale : A. 25°C B. 373°C
SOLUTION: (A) We know that temperature in
K = °C + 273
K = 25° + 273 = 298 K
(B) K = °C + 273 = 373 + 273 = 646 K
Give reasons for the following observations :
A. Naphthalene balls disappear with time without leaving any solid.
B. We can get the smell of perfume sitting several metres away.
(A) Naphthalene balls disappear with time without leaving any solid because they undergo sublimation i.e they directly change into vapours without passing through the liquid state.
(B) We can get the smell of perfume sitting several metres away due to diffusion. The perfumes contain solvent which carries pleasant smelling vapours. They diffuse quite fast and can reach a person sitting several metres away.
Arrange the following substances in increasing order of forces of attraction between the particles : water, sugar, oxygen.
SOLUTION:The forces of attraction are the strongest in solids, followed by liquids and the weakest in gases. Oxygen is a gas, water is a liquid and sugar is a crystalline solid. So the increasing order of forces of attraction is oxygen < water < sugar.
Q 22. What is the physical state of water at
A. 25°C
B. 0°C
C. 100°C?
(A) At 25°C, the physical state of water is a liquid.
(B) At 0°C, the physical state of water can be either a solid or a liquid.
(C) At 100°C, the physical state of water can be either a liquid or a gas (steam).
Give two reasons to justify that :
A. Water at room temperature is a liquid.
B. An iron almirah is a solid at room temperature.
(A) Water is a liquid at room temperature due to following reasons :
(i) Water has fixed volume, but not fixed shape, it takes the shape of the container.
(ii) Water can flow easily, hence it is not rigid but a fluid.
(B) An iron almirah is a solid at room temperature due to following reasons :
(i) An almirah has a fixed shape and a fixed volume.
(ii) It cannot flow, hence it is rigid.
Why is ice at 273 K more effective in cooling than water at the same temperature?
SOLUTION:Ice at 273 K melts to form water at 273 K by absorbing heat energy equal to latent heat from the surroundings. Hence ice has less heat energy than water and is more effective in cooling.
Q 25.Which produces more severe burns, boiling water or steam?
SOLUTION:Steam contains more heat, in the form of latent heat, than boiling water. So when steam comes in contact with skin it gives out 22.5 × 105 joules per kilogram more heat than boiling water, so steam causes more severe burns.
Q 26. Name A, B, C, D, E and F in the following diagram showing change in its state.
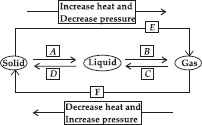
A → Fusion or melting, B → Vaporisation,
C → Condensation, D → Solidification,
E → Sublimation, F → Sublimation