Carbon and its Compounds - NCERT Questions
What would be the electron dot structure of carbon dioxide which has the formula CO2?
SOLUTION:Ans.: The atomic number (Z) for carbon is six and its electronic configuration is 2, 4. Carbon has four valence electrons. Each oxygen atom (Z = 8) has six valence electrons (2, 6). In order to complete its octet, the carbon atom shares its four valence electrons with the four electrons of the two oxygen atoms as follows :
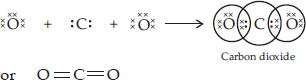
Thus, in carbon dioxide molecule, the carbon atom is linked to two oxygen atoms on both sides by two shared pairs of electrons resulting in double bonds on either sides. Both carbon and oxygen atoms complete their octet as a result of electron sharing.
What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint : The eight atoms of sulphur are joined together in the form of a ring).
SOLUTION:Ans.: The atomic number (Z) of sulphur is sixteen and its electronic configuration is 2, 8, 6. The sulphur atom has six valence electrons. The chemical formula of sulphur molecule is S8. Each sulphur atom is linked to similar atoms on either sides by single covalent bonds and thus, completes its octet. The molecule is in the form of a ring also represented by crown shape.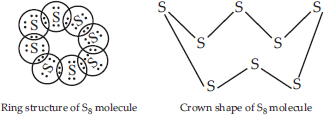
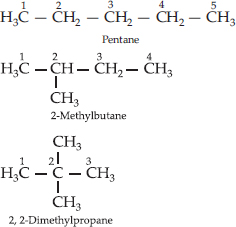
How many structural isomers can you draw for pentane?
SOLUTION:Ans.: Pentane (C5H12) has a skeleton of five carbon atoms. It can exist as a straight chain as well as two branched chains. There are three structural isomers for the hydrocarbon which is an alkane.
What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
SOLUTION:Ans.: (i) Catenation : Carbon has the unique property of self linking which is known as catenation. In fact, any number of carbon atoms can be linked to one another by covalent bonds. This is on account of the stability of C — C bonds since the size of the carbon atom is quite small.
(ii) Linking of carbon with other atoms : Carbon is tetravalent in nature and can readily unite with atoms like hydrogen, oxygen, nitrogen, sulphur etc. by electron sharing.
What will be the formula and electron dot structure of cyclopentane?
SOLUTION:Ans.: Cyclopentane is a cyclic compound with formula C5H10. The structure of the compound may be represented as :
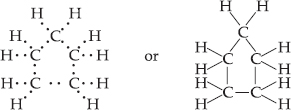
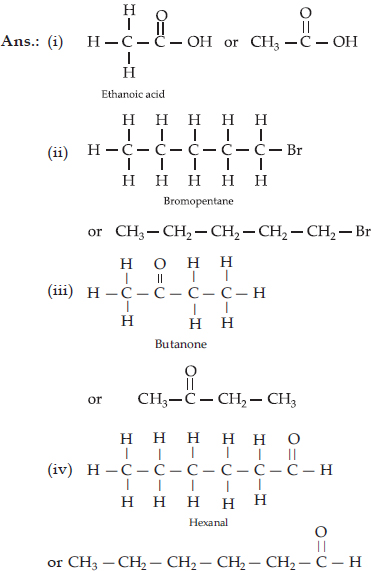
Draw the structures of the following compounds :
(i) Ethanoic acid
(ii) Bromopentane
(iii) Butanone
(iv) Hexanal
Are structural isomers possible for bromopentane?
Bromopentane has a chain of five carbon atoms. It can exist in a number of forms which are structural isomers.
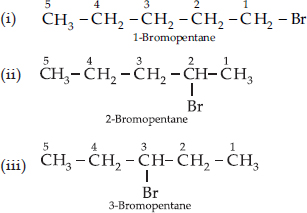
• The structural isomers (i), (ii) and (iii) which differ in the position of the Br atom are known as position isomers.
• The structural isomers (iv), (v) and (vi) which differ in the arrangement of carbon atoms in the chain are called chain isomers.
• In writing the IUPAC name, the name of prefix bromo is written before that of prefix methyl. In fact, alphabetical order is followed while naming the different prefixes.
How would you name the following compounds?
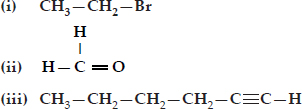
Ans.: (i) Bromoethane
(ii) Methanal
(iii) Hex-1-yne
Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
SOLUTION:Ans.: Ethanoic acid (CH3COOH) have one oxygen atom more and two hydrogen atoms less than ethanol (C2H5OH). In general.
• Loss of hydrogen is known as oxidation.
• Gain of oxygen is known as oxidation.
Therefore, it is an oxidation reaction.
A mixture of ethyne and oxygen is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
SOLUTION:Ans.: When ethyne is burnt in oxygen, large quantity of heat along with light is produced. The heat evolved can be used for gas welding which usually carried to weld small broken pieces of articles made up of iron.
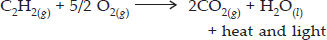
Air mainly contains a mixture of nitrogen (4 parts) and oxygen (1 part). As we known, nitrogen gas does not support combustion, this means that in air, only oxygen will help in the combustion of ethyne. Therefore, it is always better to use oxygen for the combustion of ethyne.
How would you distinguish experimentally between an alcohol and a carboxylic acid?
SOLUTION:Ans.: The distinction can be made by the following tests :
(i) Dip a strip of blue litmus separately in both alcohol and carboxylic acid taken in two glass tubes. The colour will change to red in the tube containing carboxylic acid and not in the tube which contains alcohol.
(ii) Add a small amount of solid sodium hydrogencarbonate (NaHCO3) in both the tubes. A brisk effervescence accompanied by bubbles will be noticed in the tube containing carboxylic acid and not in the tube containing alcohol.
What are oxidising agents?
SOLUTION:Ans.: Oxidising agents are the substances which either on their own or on reacting with another substance release oxygen in order to carry oxidation reactions. The commonly used oxidising agents are ozone, bromine water, a mixture of potassium dichromate and sulphuric acid or a mixture of potassium permanganate and sulphuric acid etc.
Q 12.Would you be able to check if water is hard by using a detergent?
SOLUTION:Ans.: No, it is not possible. Actually detergents produce foam in any type of water ; whether hard or soft. Therefore, a distinction between the two cannot be made. However, soaps can be used for this purpose.
Q 13.People use a variety of methods to wash clothes. Usually after adding the soap, they beat the clothes on a stone or beat them with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is this agitation necessary to get clean clothes?
SOLUTION:Ans.: The purpose of soap or detergent in washing is to reduce friction between oil drops carrying dirt particles and water so that they may mix with each other. All the methods that have been suggested loosen the bonds between the dust or oil particles and fabrics of clothes. The agitation helps in washing the clothes.
Q 14.Ethane, with the molecular formula C2H6has
A. 6 covalent bonds
B. 7 covalent bonds
C. 8 covalent bonds
D. 9 covalent bonds.
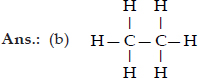
The molecule has seven covalent bonds.
Butanone is a four carbon compound with the functional group
A. carboxylic acid
B. aldehyde
C. ketone
D alcohol.
Ans.: (c)The functional group is ketone ( > C  O) also known as alkanone.
O) also known as alkanone.
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
A. the food is not cooked completely
B. the fuel is not burning completely
C. the fuel is wet
D. the fuel is burning completely.
Ans.: (B) The fuel is not burning completely. The unburnt particles present in smoke blacken the vessel from outside.
Q 17.Explain the nature of the covalent bond using the bond formation in CH3Cl.
SOLUTION:Ans.: The molecule of chloromethane (CH3Cl) consists of three elements i.e., carbon (Z = 6) hydrogen (Z = 1) and chlorine (Z = 17). Carbon atom has four valence electrons (2, 4) ; hydrogen has one (1) while chlorine has seven electrons in the valence shell (2, 8, 7). In order to complete its octet, carbon shares three valence electrons with three hydrogen atoms while one is shared with the electron of chlorine atom. The structure of covalent molecule may be written as follows :
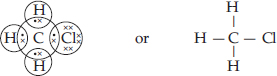
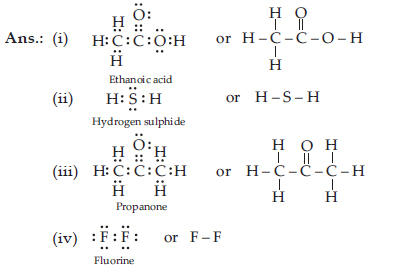
Draw the electron dot structures for
(i) Ethanoic acid
(ii) H2S
(iii) Propanone
(iv) F2
What is a homologous series? Explain with an example.
SOLUTION:Ans.: A homologous series can be defined as a family of organic compounds having the same functional group, similar chemical properties and the successive members of which differ by a – CH2 group or 14 mass units. For example, CH3OH (methanol), CH3CH2OH (ethanol), CH3CH2CH2OH (propanol), CH3CH2CH2CH2OH (butanol), etc. constitute a homologous series of alcohols. They have the same functional group, i.e., OH (hydroxyl). Since they have the same functional group, they show similar chemical properties. The difference between any two successive members is a CH2 group or 14 mass units. Their physical properties such as melting point and boiling point increase as the molecular mass increases. Their solubility in water, however, decreases with increase in molecular mass.
Q 20.How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
SOLUTION:Ans.: Distinction based on physical properties :
(i) Smell : Ethanol has a characteristic smell known as alcoholic smell which is pleasant. Ethanoic acid has vinegar like smell.
(ii) Boiling points : Boiling point of ethanol (351 K) is less than that of ethanoic acid (391 K).
(iii) Litmus test : Ethanol is neutral in nature and does not bring any change in the colour of litmus whether blue or red. Ethanoic acid is acidic and changes the colour of a blue litmus strip to red when dipped in it.
Distinction based on chemical properties :
(i) Action with sodium hydrogencarbonate : On adding a small amount of sodium hydrogencarbonate to ethanoic acid, carbon dioxide gas is evolved with brisk effervescence. However, no such reaction is noticed in case of ethanol.
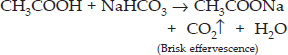
(ii) Action with caustic alkalies : Ethanoic acid reacts with both sodium hydroxide (NaOH) and potassium hydroxide (KOH) to form corresponding salt and water. Ethanol fails to react with either of these.
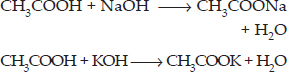
Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
SOLUTION:Ans.: Soap may be represented by the formula RCOO–Na+ where R is an alkyl group which represents long chain of carbon with fifteen or more atoms. Oil drops containing dirt particles and water do not mix. Soap helps in their mixing by reducing interfacial tension or friction. Actually it forms a sort of bridge between oil drops and water in which the alkyl portion (hydrophobic end) point towards oil drop while other portion COO–Na+ (hydrophilic end) is directed towards water. This is known as micelle formation. Thus, soap helps in the formation of a stable emulsion between oil and water. Ethanol and other similar solvents which are of organic nature do not help in micelle formation because soap is soluble in them.
Q 22.Why are carbon and its compounds used as fuels for most applications?
SOLUTION:Ans.: Carbon burns in oxygen or air to form carbon dioxide gas. The reaction is highly exothermic. That is why different forms of coal are used as fuels. The most important compounds of carbon are hydrocarbons. Just like carbon, hydrogen also readily burns in oxygen or air to form water producing heat. The hydrocarbon methane (CH4) is a constituent of natural gas. Propane (C3H8) and butane (C4H10) are present in liquid petroleum gas (L.P.G.). Petrol and kerosene also contain different hydrocarbons. Therefore, these are used as fuels.
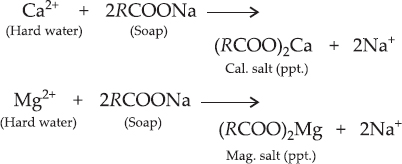
Q 23.Explain the formation of scum when hard water is treated with soap.
SOLUTION:Ans.: Soap is basically sodium or potassium salt of higher fatty acid. Hard water contains in it Ca2+ and Mg2+ ions as their salts. When soap is added to hard water, the corresponding calcium and magnesium salts are formed. These are in the form of precipitates, also called 'scum'.
What change will your observe if you test soap with litmus paper (blue or red)?
SOLUTION:When soap is dissolved in water, the solution is alkaline in nature due to the formation of alkali NaOH or KOH. The solution changes the colour of red litmus to blue. However, the solution does not change the colour of blue litmus. Q 25.What is hydrogenation? What is its industrial application?
SOLUTION:Ans.: Addition of hydrogen to an unsaturated carbon compound is called hydrogenation reaction. In industry, hydrogenation reaction is used for preparing vegetable ghee from vegetable oils. Vegetable oils such as groundnut oil, cottonseed oil, which contain double bonds in their molecules, are converted into ghee by hydrogenation in the presence of Ni. The process of converting a vegetable oil into a solid fat (vegetable ghee) is called hydrogenation of oil.
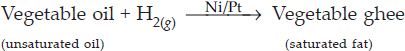
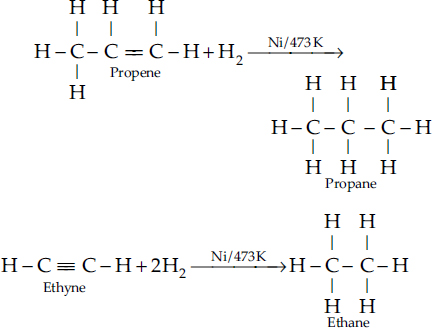
Which of the following hydrocarbons undergo addition reactions : C2H6, C3H8, C3H6, C2H2 and CH4?
SOLUTION:Ans.: In order that a hydrocarbon may undergo addition reaction, it must be unsaturated in nature. It must be either an alkene (C  C) with general formula CnH2n or an alkyne (C
C) with general formula CnH2n or an alkyne (C C) with general formula CnH2n–2. Out of the list of the hydrocarbons given :
C) with general formula CnH2n–2. Out of the list of the hydrocarbons given :
• C3H6 (Propene) is an alkene with C  C bond. It corresponds to general formula CnH2n (n = 3).
C bond. It corresponds to general formula CnH2n (n = 3).
• C2H2 (Ethyne) is an alkyne with C  C bond. It corresponds to general formula CnH2n–2 (n= 2).
C bond. It corresponds to general formula CnH2n–2 (n= 2).
Both these hydrocarbons take part in addition reaction. For example, they react with hydrogen upon heating to 473 K in the presence of nickel catalyst to form corresponding alkanes.
Give a test that can be used to differentiate chemically between butter and cooking oil?
SOLUTION:Ans.: Butter is saturated in nature while cooking oil is unsaturated. This means that cooking oil has at least one C C bond present in the constituting compounds while butter does not have any such bond. The distinction between them can be made by reacting with bromine water or bromine dissolved in carbon tetrachloride. Cooking oil will discharge the yellow colour of bromine while butter will not.
C bond present in the constituting compounds while butter does not have any such bond. The distinction between them can be made by reacting with bromine water or bromine dissolved in carbon tetrachloride. Cooking oil will discharge the yellow colour of bromine while butter will not.
Explain the mechanism of cleansing action of soaps.
SOLUTION:Ans.: When an oily (dirty) piece of cloth is put into soap solution, the hydrocarbon part of the soap molecule attaches itself to the oily drop, and the —COO– end orients itself towards water. The Na+ ions in solution arrange themselves around the —COO– ions. The negatively charged micelle so formed entrap the oily dirt. The negatively charged micelles repel each other due to the electrostatic repulsion. As a result, the tiny oily dirt particles do not come together and get washed away in water during rinsing.