Combustion and Flame (Chemistry) Class 8 - NCERT Questions
List conditions under which combustion can take place.
SOLUTION:The conditions necessary for combustion to take place are as follows:
(A) Presence of a combustible substance.
(B) Presence of supporter of combustion.
(C) Heating the combustible substance to its ignition temperature.
Combustion will not occur in the absence of any one of these.
Fill in the blanks.
(A) Burning of wood and coal causes ............. of air.
(B) A liquid fuel, used in homes is .............. .
(C) Fuel must be heated to its ................. ............ before it starts burning.
(D) Fire produced by oil cannot be controlled by ............... .
(A) pollution
(B) kerosene
(C) ignition temperature
(D) water
Explain how the use of CNG in automobiles has reduced pollution in our cities.
SOLUTION:The use of CNG in place of petrol and diesel reduce pollutions in following ways:
(i) It produces less carbon monoxide gas.
(ii) It produces less carbon dioxide gas.
(iii) It produces less amount of sulphur dioxide and nitrogen dioxide which cause acid rain.
(iv) No residue remain after combustion.
Compare LPG and wood as fuels.
SOLUTION: 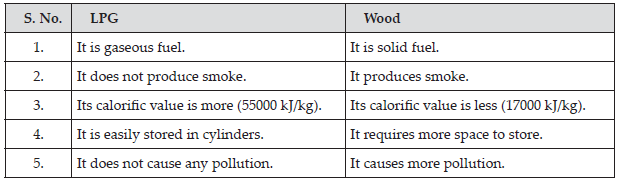
Give reasons :
(A) Water is not used to control fires involving electrical equipment.
(B) LPG is a better domestic fuel than wood.
(C) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
(A) Water is a good conductor of electricity. It conducts electricity and may result electric shock.
(B) LPG has more calorific value than wood and LPG produces no pollution. So LPG is a better domestic fuel than wood.
(C) The ignition temperature of paper is less, so it catches fire easily. It does not catch fire when wrapped around aluminium pipe because aluminium absorbs the heat so paper does not attain its ignition temperature.
Make a labelled diagram of candle flame.
SOLUTION: 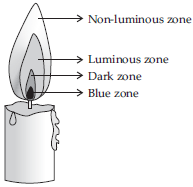
Name the unit in which the calorific value of a fuel is expressed.
SOLUTION:The unit of calorific value is kilojoule per kilogram (kJ/kg).
Q 8.Explain how CO2 is able to control fire.
SOLUTION:CO2 forms a blanket around the fire, cutting off the air supply, due to which the fire gets extinguished.
Q 9.It is difficult to burn a heap of green leaves but dry leaves catches fire easily. Explain.
SOLUTION:The green leaves contain some water due to which the ignition temperature of leaves increases and they do not catch fire easily while dry leaves have no water so they catch fire easily.
Q 10.Which zone of a flame does a goldsmith use for melting gold and silver and why?
SOLUTION:A goldsmith uses the non-luminous zone (outermost zone) of candle flame to melt gold and silver because it is the hottest part of the candle flame and has most temperature.
Q 11.In an experiment 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
SOLUTION:Total mass of fuel = 4.5 kg
Total heat produced = 180,000 kJ
Heat produced by burning 1 kg of fuel = 180,000
kJ/4.5 kg = 40,000 kJ/kg.
So, calorific value of fuel = 40,000 kJ/kg.
Can the process of rusting be called combustion? Discuss.
SOLUTION:The process of rusting cannot be called combustion because in this process no heat and light is produced. Due to this reason iron is not considered as combustible substance.
Q 13.Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water will get heated in a shorter time?
SOLUTION:The water heated by Ramesh will get heated in a shorter time because he kept his beaker near the hottest zone of the flame.